An infrared spectroscopy correlation table or table of infrared absorption frequencies is a list of absorption peaks and frequencies typically reported in wavenumber for common types of molecular bonds and functional groups. Some time later the molecule gives up this extra energy by emitting another infrared photon.

Infrared Spectroscopy Pramod K Singh School Of Basic
Infrared
Principles Of Infrared Spectroscopy 1 Molecular Vibrations And Infrared Absorption Jasco Global
On the immediate high energy side of the visible spectrum lies the ultraviolet and on the low energy side is the infrared.
Molecule absorb infrared. A infrared IR radiation causes covalent bonds to vibrate more and absorb energy. The peaks in infrared spectra are caused by absorption of characteristic frequencies by molecules. An important observation made by early researchers is that many functional group absorb infrared radiation at about the same wavenumber regardless of the structure of the rest of the molecule.
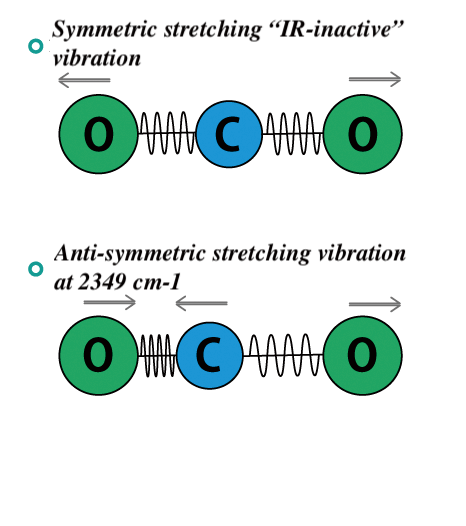
4 There are three general vibrations for a CO 2 molecule. The molecule gains kinetic energy by. The infrared radiation strikes a molecule such as carbon dioxide and causes the bonds to bend and vibrate - this is called the absorption of IR energy.
The energy from the photon causes the CO 2 molecule to vibrate. The portion of the infrared region most useful for analysis. Concentrations of CFC gases in the atmosphere are the highest of any of the halocarbons and they can absorb more infrared radiation than any other greenhouse gas.
The majority of the classical analytical methods rely on chemical reactions to perform an analysis. THEORY When a molecule absorb radiation with a frequency less than 100 cm-1 molecular rotation takes place and if a molecule absorb more energetic radiation in the region of 104 to 102 cm-1 molecular vibration takes place. Different functional groups of a molecule like CH3 COOH NH2 exhibit peaks at different ranges of wavenumbers.
Each mode is able to absorb certain bands of wavelengths with the bending mode absorbing longer wavelengths 667 cm-1 and the asymmetric absorbing shorter wavelengths 2349 cm-1 Figure 2. Spectra-structure correlations are shown in a chart form where one can look for the groups that absorb in a given region or. A single vibrational energy change is accompanied by a large number of rotational energy changes and thus the vibrational spectra appear as vibrational rotational bands.
Infrared IR spectroscopy is. In quantum physics organic chemistry and biochemistry the distinction from ions is dropped and molecule is often used when referring to polyatomic ions. It induces stronger molecular vibrations in covalent bonds which can be viewed as springs holding together two masses or atoms.
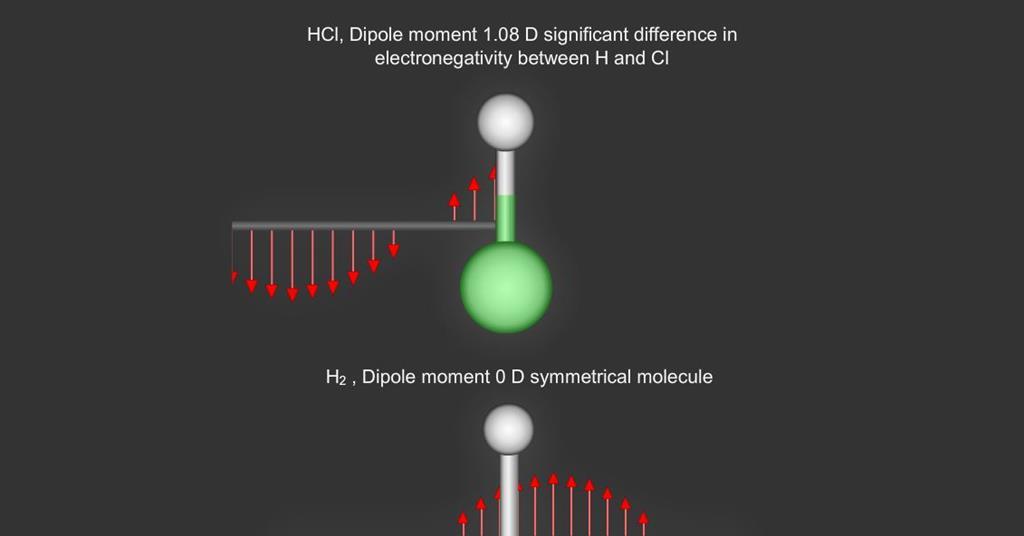
The greater the change in charge distribution the stronger the absorption. Theory of Infrared Absorption At temperatures above absolute zero all the atoms in molecules are in continuous vibration with respect to each other. Some bonds absorb more strongly than others and some compounds give very complex spectra.
Infrared Spectroscopy and Mass Spectrometry Introduction It is fundamental for an organic chemist to be able to identify or characterize the new compound that heshe has just made. In practice infrared spectra do not normally display separate absorption signals for each of the 3n-6 fundamental vibrational modes of a molecule. It uses gentle red-light phototherapy with 635nm waves to slim the body and whittle away stubborn areas of.
Classical qualitative analysis is performed by adding one or a series of chemical reagents to the analyte. When the frequency of a specific vibration is equal to the frequency of the IR radiation directed on the molecule the molecule absorbs the radiation. The impact of 1 molecule of a CFC gas is equivalent to 10000 molecules of carbon dioxide.
The Nushape Lipo Wrap is the first home-use red light therapy treatment of its kind. In general a vibration must cause a change in the charge distribution within a molecule to absorb infrared light. For example C-H stretching vibrations usually appear between 3200 and 2800cm -1 and carbonylCO stretching vibrations usually appear between 1800 and 1600cm -1.
All photosynthetic organisms plants certain protistans prochlorobacteria and cyanobacteria have chlorophyll a. Chemical analysis - chemical analysis - Classical methods. When infrared light is incident on the molecule the frequency which corresponds withthe natural vibrational frequency may beabsorbed by the molecule increasing the magnitude of the natural molecular vibrations.
Chlorophyll is a complex molecule. Sometimes this can be achieved by a chemical means such as determining the elemental composition and molecular weight. Bonds in a molecule absorb infrared radiation at characteristic wavenumbers.
Molecules are distinguished from ions by their lack of electrical charge. The first route occurs when absorption of radiation leads to a higher rotational energy level in a rotational transition. And each of these routes involves an increase of energy that is proportional to the light absorbed.
Introduction The light our eyes see is but a small part of a broad spectrum of electromagnetic radiation. CO 2 molecules absorb infrared light at a few wavelengths but the most important absorption is light of about 15 microns says Kroll. IR Spectroscopy Instrumentation The instrumentation of infrared spectroscopy is illustrated below.
The number of observed absorptions may be increased by additive and subtractive interactions leading to combination tones and overtones of the fundamental vibrations in much the same way that sound vibrations from a musical instrument interact. Molecules of carbon dioxide CO 2 can absorb energy from infrared IR radiation. Those absorptions are caused by different vibration modes of a molecule.
In the kinetic theory of gases the term molecule is often used. 5 Infrared radiation is largely thermal energy. There are three main processes by which a molecule can absorb radiation.
Several modifications of chlorophyll occur among plants and other photosynthetic organisms. Incoming light from the sun tends to have much shorter wavelengths than this so CO 2 doesnt stop this sunlight from warming the Earth in the first place. A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.
A symmetric mode a bending mode and an asymmetric mode Figure 1. This animation shows a molecule of CO 2 absorbing an incoming infrared photon yellow arrows. Molecule increases however.
In physical and analytical chemistry infrared spectroscopy IR spectroscopy is a technique used to identify chemical compounds based on the way infrared. Organic Chemistry 5th ed. For instance the molecule can absorb the energy contained in the incident light and the result is a faster rotation or a more pronounced vibration.
42 Alcohols haloalkanes and analysis. Infrared radiation was discovered by. Pearson Education Inc 2003 Specific bonds respond to absorb specific frequencies VIBRATIONAL MODES.
Not all molecular vibrations lead to observable infrared absorptions. It is based on absorption spectroscopy 5. In contrast instrumental methods typically depend on the measurement of a physical property of the analyte.
Infrared spectroscopy IR spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum that is light with a longer wavelength and lower frequency than visible light Infrared Spectroscopy is the analysis of infrared light interacting with a molecule.

Earth S Greenhouse Gases Blog E Bu Utami
Umdberg Spectroscopy How Does Light Interact With Matter

Properties American Chemical Society

Geo Expro Recent Advances In Climate Change Research Part Viii How Carbon Dioxide Absorbs Earth S Ir Radiation
Why Does Carbon Dioxide Absorb Infrared Radiation Quora
Infrared Ir Spectroscopy Energy Levels Resource Rsc Education

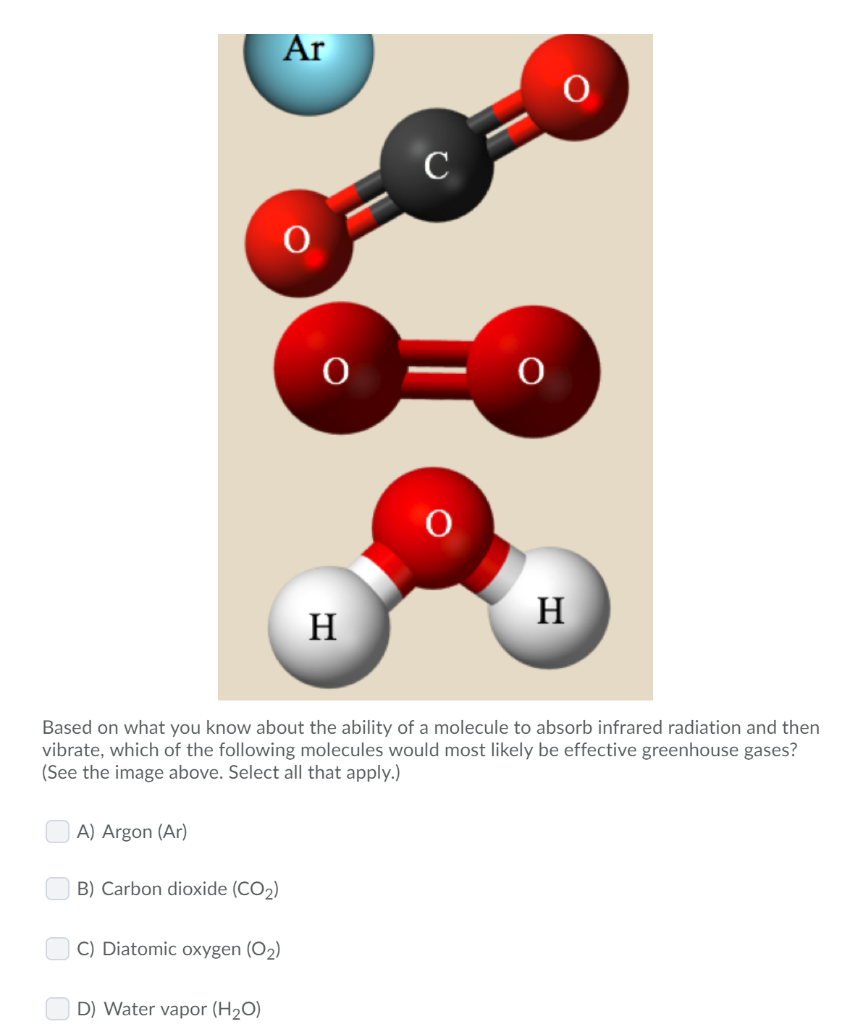
Solved Ar Based On What You Know About The Ability Of A Chegg Com
Infrared Spectroscopy
