Then each supplement is assigned a score from 0 to 10 which enables you to easily compare the effectiveness of different brands. The site of action or the bodily fluid domain from which the drugs intended targets have unimpeded access123 For majority.
Bioavailability Absolute Bioavalability Relative Bioavailability
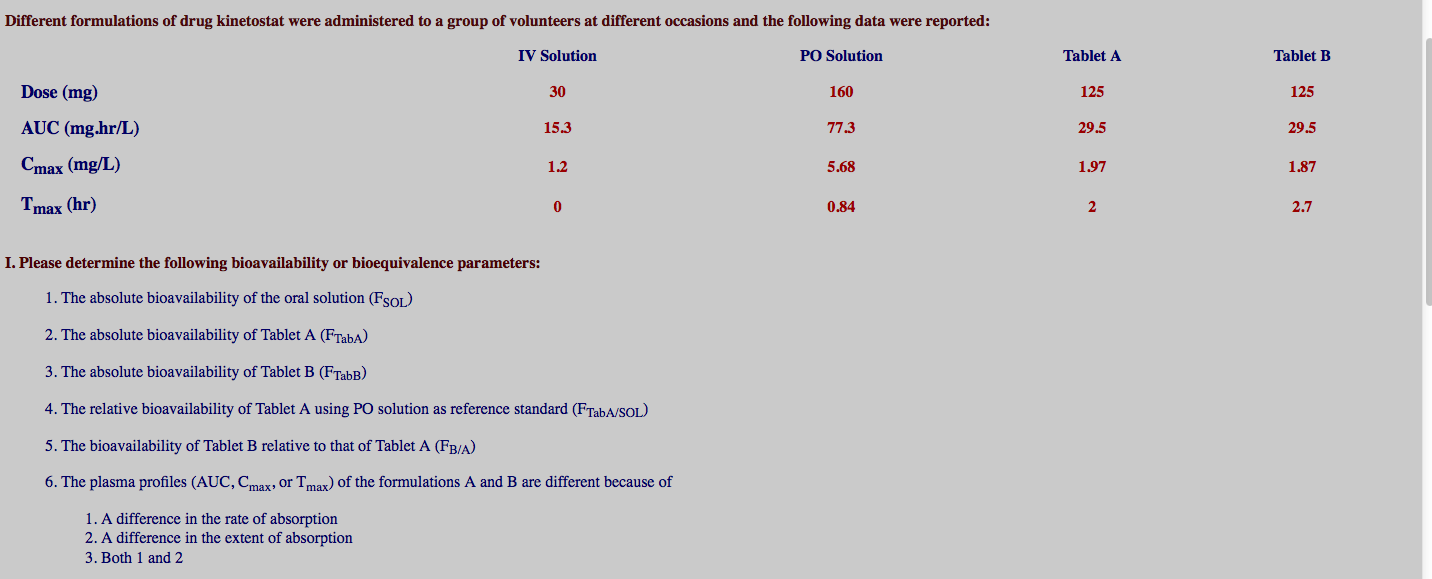
Solved Different Formulations Of Drug Kinetostat Were Chegg Com

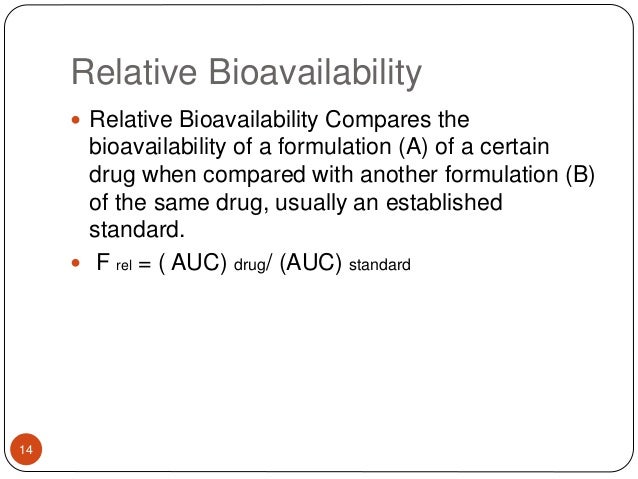
Bioavailability Absolute Bioavalability Relative Bioavailability
74 of the impact of the route of administration on BA and defines the absolute BA of the drug.
What is absolute bioavailability. Do not initiate XELJANZXELJANZ XR if absolute lymphocyte count absolute neutrophil count ANC. At K9 Power we know your dog is family. If the absolute bioavailability of the oral capsule is determined to be 074 calculate the absolute bioavailability of the tablet dosage form.
The draft guidance provides recommendations to sponsors andor applicants planning to include bioavailability BA and bioequivalence BE information for drug products in. 22 Recommended dosage in patients with moderate and severe renal. In order to view PDF documents you must install the Adobe Acrobat Reader which is.
CBD oil nanodroplets fuse with water much better and easily penetrate your cells resulting in absolute bioavailability. The low systemic availability is attributed to presystemic clearance in gastrointestinal mucosa andor hepatic first. The bioavailability of the best water-soluble CBD oil reaches 90.
The information on this site is the culmination of over 6 years of scientific research and analysis. Based on the bioequivalence studies under fasting and fed conditions Idiazole Rabeprazolnatrium and Rodesa 20 mg tablet are bioequivalent with Pariet 20 mg. Two medicines are bioequivalent if there is no clinically significant difference in their bioavailability.
Oral tablet suppository subcutaneous etc compared with the bioavailability of the same drug administered intravenously IV. The uptake of Mg 2 can be influenced by physiological factors such as age and the other food components. The job listings are posted in Adobe Acrobat PDF format only.
A is used as a subscript for pharmacokinetic parameters appropriate to the distributive phase eg t 12a V da etc. RESEARCH RANDOMIZER RESEARCH RANDOMIZER RANDOM SAMPLING AND RANDOM ASSIGNMENT MADE EASY. Women a smaller apparent volume of distribution adjusted for weight and higher absolute bioavailability resulted in higher plasma ondansetron levels.
21 Recommended Dosage. Bioavailability can be measured in terms of absolute bioavailability or relative bioavailability. Bioavailability uptake metabolism storage and excretion of chemicals constitute toxicokinetics.
The bioavailability of calcium refers to the fraction of dietary calcium that is potentially absorbable and the incorporation of the absorbed calcium into bone. This website uses cookies to help provide you with the best possible online experience. B Compartments Volume of Distribution Half-Life.
Bioavailability refers to the extent a substance or drug becomes completely available to its intended biological destinations. More accurately bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either. The total amount excreted into urine as unchanged drug after an oral tablet A of 75 mg was 486 mg.
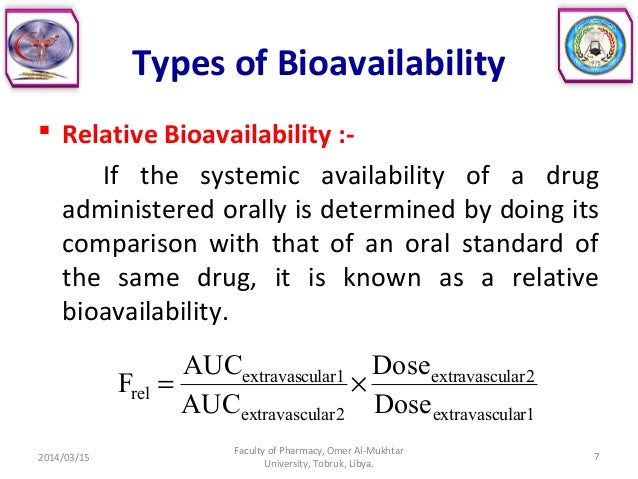
79 It may be useful to distinguish between the absolute bioavailability of a given dosage form. Comparison of the plasma concentration-time profiles of the drug between the test and reference products containing the same amount of the same active ingredients provides an estimate of relative. Absolute bioavailability compares the bioavailability of the active drug in systemic circulation following non-intravenous administration ie after oral buccal ocular nasal rectal transdermal subcutaneous or sublingual administration with the bioavailability of the same drug following intravenous administrationIt is the fraction of the drug absorbed through non-intravenous.
The absolute bioavailability of atorvastatin parent drug is approximately 14 and the systemic availability of HMG-CoA reductase inhibitory activity is approximately 30. These higher plasma levels may in part be explained by differences in body weight between men and women. Ethanol absolute CAS 64-17-5 for analysis EMPARTA ACS - Find MSDS or SDS a COA data sheets and more information.
Research Randomizer is a free resource for researchers and students in need of a quick way to generate random numbers or assign participants to experimental conditions. Or hemoglobin. Rheumatoid Arthritis XELJANZ 5 mg twice daily or XELJANZ XR 11 mg once daily.
In principle the relative bioavailability of Mg 2 is higher when the mineral is taken up in multiple low doses throughout the day compared to a single intake of a high amount of Mg 2. I hope this article prepares you with the best starting knowledge out there the ideal N-P-K numbers for your needs what exact amendment is your best bet in any given situation and even comparisons between the benefits of organic versus inorganic fertilizers. Its why we add a seat at the table for them nutritionally speaking.
76 -Bioavailability is understood to be the extent and the rate to which a substance or its 77 active moiety is delivered from a pharmaceutical form and becomes available in the 78 general circulation. However absolute absorption increases with the dose. Each of the reviewed products is evaluated against 4 key parameters such as composition bioavailability and potency.
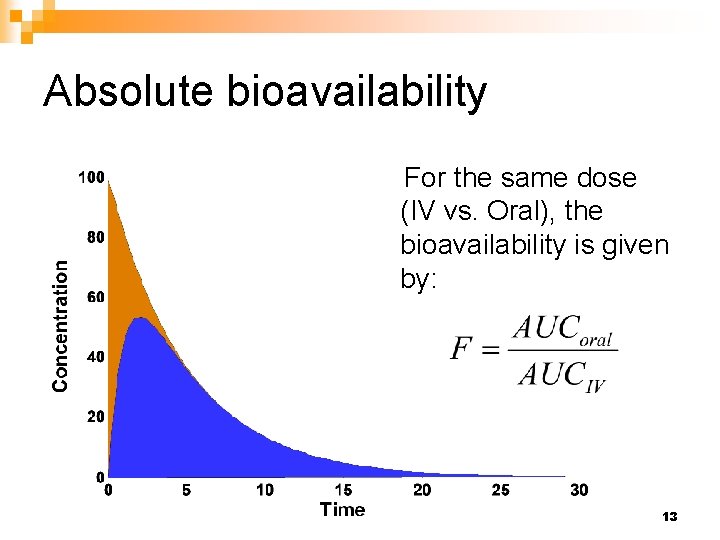
Comparison of the AUC values following oral versus intravenous administration of an equivalent dose of the same active ingredient provides an estimate of absolute bioavailability for most drugs. It is not known whether these gender-related differences were clinically important. RANDOM SAMPLING AND RANDOM ASSIGNMENT MADE EASY.
Our products are designed to enhance overall health so they can live their most complete lives. Please read our Terms Conditions and Privacy Policy for information about. To meet calcium recommendations the bioavailability of calcium is an important factor to consider beyond simply the calcium content of foods.
The earlier segment of a biphasic plot of log C against t following intravenous injection of a drug represents the distributive phase of a drugs sojourn in the body. Absolute bioavailability edit Absolute bioavailablity refers to the bioavailability of drug when administered via a non-intravenous non-IV dosage form ie. In fact using them is completely necessary an absolute requirement in a good number of instances.
Thus switching to nano CBD is an excellent way to deliver the whole cannabidiol serving to your bloodstream. Bioavailability is the potential for uptake of a substance by a living organism. The right of access is not absolute as a court may limit access to court records in certain situations.
So the absolute bioavailability of this drug product is 52 7. Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs. It is usually expressed as the fraction that can be taken up by the organism in relation.
The New Jersey Court Rule 138 covers the right of public access to the court records.

Chapter 10 Page 4

In Vivo Assessment Of Arsenic Bioavailability In Rice And Its Significance For Human Health Risk Assessment Environmental Health Perspectives Vol 114 No 12

Intrinsic Absolute Bioavailability Prediction In Rats Based On In Situ Absorption Rate Constants And Or In Vitro Partition Coefficients 6 Fluoroquinolones Journal Of Pharmaceutical Sciences

Drug Bioavailability Prof Dr Basavaraj K Nanjwade M Pharm Ph D Ppt Download

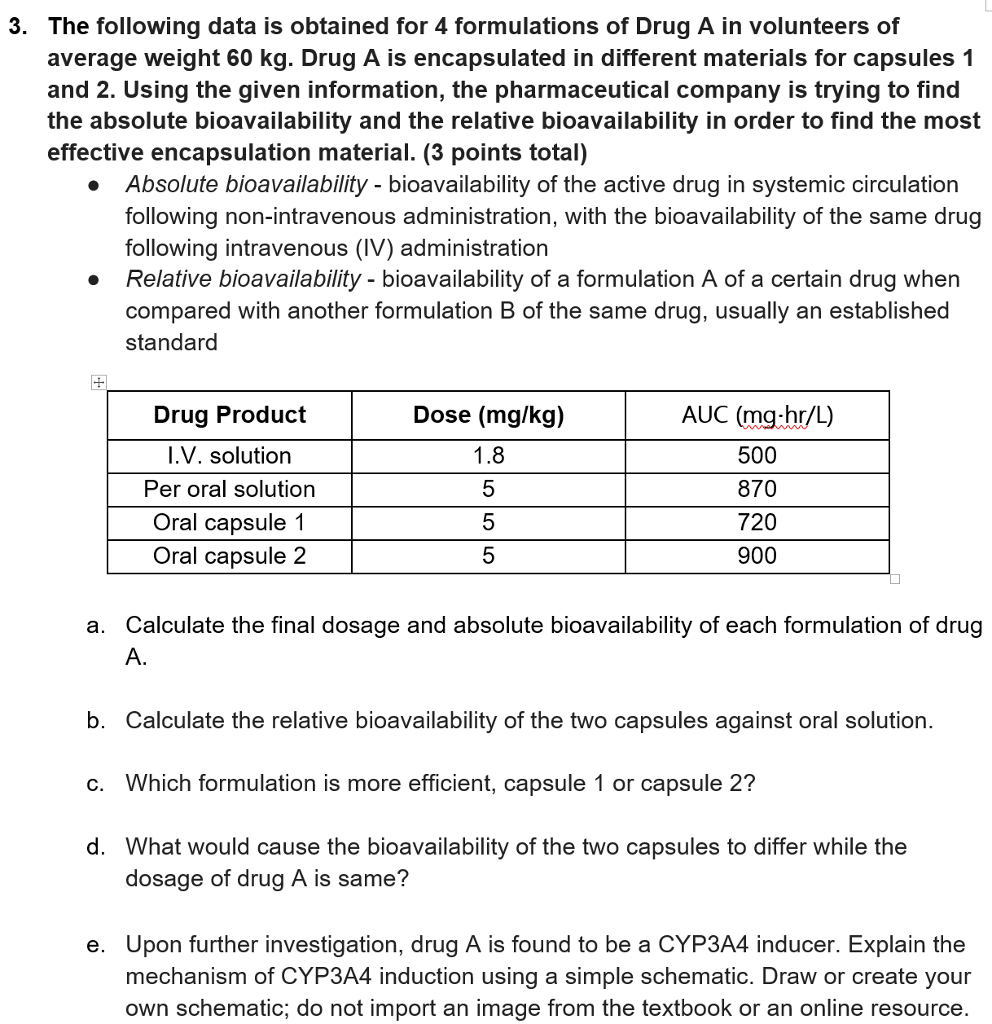
3 The Following Data Is Obtained For 4 Formulations Chegg Com

Bioavailability 1 Intro Absolute Comparative Bioav Fix Pharma
Bioavailability Dr Mohammad Issa 1 Which Formulation Has
Bioavailability
